October 10 - 16, 2021: Issue 513
Landmark Trial Eliminates Pest Mosquito

By CSIRO
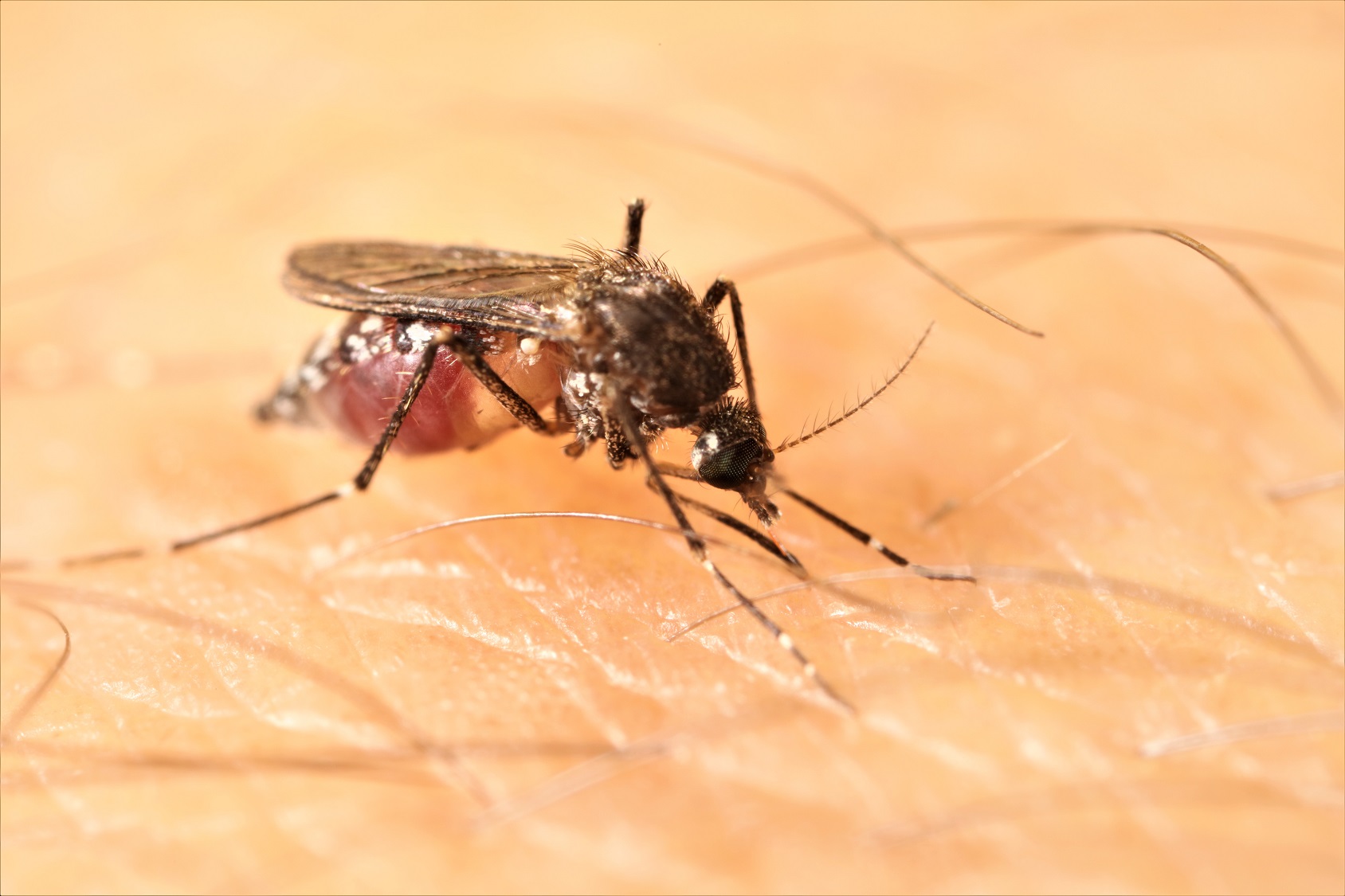
In a first for the Southern Hemisphere, researchers have shown a bacteria can successfully sterilise and eradicate the invasive, disease carrying Aedes aegypti mosquito which is responsible for spreading dengue, yellow fever and Zika.
The breakthrough could support the suppression and potential eradication of Aedes aegypti worldwide.
Published today in PNAS, the landmark trial involved releasing three million male Aedes aegypti mosquitoes in Northern Queensland, sterilised with bacteria called Wolbachia, across three trial sites over a 20-week period during the summer of 2018. The sterile male insects search out and mate with wild females, preventing the production of offspring.
Scientists returned the following year and found one of the trial sites, Mourilyan in Queensland, was almost devoid of mosquitoes.
The trial was an international collaboration between Australia’s national science agency CSIRO, University of Queensland (UQ), Verily Life Sciences, QIMR Berghofer Medical Research Institute and James Cook University (JCU).
CSIRO Chief Executive Dr Larry Marshall said the organisation was proud to build on a 100-year legacy of protecting Australia and Australians.
“The creation of our Health and Biosecurity team back in 2016 meant we were prepared for COVID-19, and that preparation is paying off across other biosecurity threats like mosquitos which spread some of the world’s most serious diseases,” Dr Marshall said.
“Over 40 per cent of humans suffer from mosquito-spread diseases, so it’s an opportunity for Australia to develop environmentally-friendly mosquito control tools to tackle current and future mosquito incursions.
“By working with Australian and international partners we can tackle two of Australia’s greatest challenges at once – health and security – with breakthrough research translated into effective global export solutions.
“CSIRO is leveraging great Australian science to create new technologies to make this approach more cost effective and suitable for the climates of less developed countries that suffer most from mosquito-borne viruses, strengthening and protecting our region.”
JCU Adjunct Professor Scott Ritchie said the Wolbachia trial was a successful international collaboration which saw contemporary science working together with cutting-edge technology, to help eliminate the dengue-carrying Aedes aegypti mosquito.
“It was a hugely successful project. We reared the three million male Aedes aegypti mosquitoes needed for the trial in the insectary at James Cook University in Cairns,” Prof Ritchie said.
Verily Product Manager, Nigel Snoad, said community engagement was also essential to success of the project.
“It was a huge achievement by the joint team to setup and operate the mosquito rearing, sorting and release systems, and develop strong community engagement and support,” Mr Snoad said.
“We were proud of the work we were able to do in collaboration with the CSIRO, James Cook University and the local community. The ongoing suppression after releases stopped is an important result, indicating that sustained impact is feasible for this disease vector.”
CSIRO scientist and UQ Associate Professor, Nigel Beebe, said the trial demonstrates this technique is robust and capable of effectively suppressing mosquito populations.
“During the trial, we saw over 80 per cent of the mosquito population suppressed across our three trial sites,” Associate Professor Beebe said.
“When we surveyed the sites the following year, we were very encouraged to see the suppression still in effect, with one of our most productive towns for Aedes aegypti almost devoid of this mosquito with a 97 per cent reduction across the following season.
“One year on, the mosquito population at the second trial site remained substantially suppressed, while the population fully recovered at the third site.
“We are currently investigating the differences observed in the following mosquito season as they are incredibly informative in further developing this technology and in modelling how we could remove this exotic virus-transmitting pest in other locations worldwide.”
The technique can also be used to remove the virus-transmitting Asian tiger mosquito, Aedes albopictus, that has now established at Australia’s doorstep in the Torres Strait Islands.
Techniques from the trial are being used to support CSIRO-led mosquito suppression programs in French Polynesia and the Hunter region in New South Wales, Australia.
Nigel W. Beebe, Dan Pagendam, Brendan J. Trewin, Andrew Boomer, Matt Bradford, Andrew Ford, Catherine Liddington, Artiom Bondarenco, Paul J. De Barro, Joshua Gilchrist, Christopher Paton, Kyran M. Staunton, Brian Johnson, Andrew J. Maynard, Gregor J. Devine, Leon E. Hugo, Gordana Rasic, Helen Cook, Peter Massaro, Nigel Snoad, Jacob E. Crawford, Bradley J. White, Zhiyong Xi, and View ORCID Scott A. Ritchie. Releasing incompatible males drives strong suppression across populations of wild and Wolbachia-carrying Aedes aegypti in Australia. PNAS October 12, 2021 118 (41) e2106828118; https://doi.org/10.1073/pnas.2106828118
Scientists Target 'Ross River' Mosquito

World’s first mass malaria vaccine rollout could prevent thousands of children dying
Danielle Stanisic, Griffith University and Michael Good, Griffith UniversityThe world’s first mass vaccination program against malaria, announced this week, is set to prevent millions of children from catching malaria and thousands dying from this debilitating disease.
The World Health Organization (WHO) has recommended widespread use of the RTS,S/AS01 (Mosquirix) vaccine in young children who are most at risk of malaria in Africa.
Malaria Is A Big Deal
Mosquitoes spread the parasite Plasmodium falciparum from person to person when they bite. So until now, our fight against malaria has involved using mosquito nets to avoid being bitten and spraying insecticide to kill mosquitoes. Then there are drugs to prevent or treat malaria infection.
However, the parasite has developed resistance to antimalarial drugs and mosquitoes have developed resistance to insecticides. Nevertheless existing control measures have resulted in a significant decrease in the number of malaria deaths since 2000.
In recent years, however, progress has stalled. In 2019, malaria infection resulted in 409,000 deaths around the world, mostly in children under five years old, and 229 million new malaria cases.

So we need extra tools, such as an effective malaria vaccine, if we are to control the disease globally.
WHO’s recommendation to roll out the Mosquirix vaccine to children at high risk of infection with P. falciparum, which is widespread in Africa, is an important step towards controlling the deadliest of human malaria parasites.
Read more: How our red blood cells keep evolving to fight malaria
What Did The WHO Recommend?
The WHO recommended four doses of the vaccine in children from five months old.
This recommendation follows recent results from a pilot program in Ghana, Kenya and Malawi, involving vaccinating more than 800,000 children since 2019.
The program showed delivering the vaccine is feasible and cost-effective in high-risk areas. It also increased the number of children (to more than 90%) who have access to at least one intervention to prevent malaria.
The vaccine has a good safety profile and reduces cases of clinical and severe malaria, which can be deadly.
Read more: New malaria vaccine proves highly effective – and COVID shows how quickly it could be deployed
What Do We Know About The Vaccine?
Mosquirix is a “subunit” vaccine. This means it only contains a small part of the malaria parasite, which is produced as a synthetic protein.
This protein is coupled with an “adjuvant”, a molecule designed to stimulate a strong immune response.
The vaccine works mainly by stimulating the body to make antibodies against the parasite, neutralising it, and preventing it from entering liver cells. These are the first cells the parasite invades when it enters the body.
The vaccine also works by helping to mount an inflammatory response, when a different part of the immune system responds.
Read more: Male mosquitoes don't want your blood, but they still find you very attractive
The Vaccine Isn’t Perfect
The level of protection the vaccine provides isn’t ideal. Protection varies with the age of the child when vaccinated, with less protection for young infants compared with older children. In the older children (5-17 months old), this averaged at about 36% protection against developing clinical malaria over a four-year period.
Protective immunity also decreases rapidly over time. This means regular booster doses will be required. Alternative immunisation schedules are also being evaluated.
Yet, the vaccine can still make a significant contribution to malaria control when used in areas of high malaria risk and with other control measures.
One modelling study estimated that in sub-Saharan Africa, Mosquirix could prevent up to 5.2 million cases of malaria and 27,000 deaths in young children each year.
Why Has It Taken So Long To Get Here?
Developing a malaria vaccine is challenging. Technically, it is difficult to develop a vaccine against a parasite that lives in two hosts (mosquitoes and humans).
There has also been limited interest by pharmaceutical companies in developing a malaria vaccine.
Although travellers would benefit from a vaccine when travelling to affected countries, the people who most need a malaria vaccine live in some of the world’s poorest countries. So there is little financial incentive to develop a vaccine.
Mosquirix is the result of more than 30 years of research and was created through a partnership between GlaxoSmithKline (GSK) and the Walter Reed Army Institute of Research in the USA.
This time-frame is not long considering both the antigen design and the adjuvant system were novel.
The Bill & Melinda Gates Foundation and GSK supported further development, including evaluating the vaccine in clinical trials. Over three decades, they invested around US$700,000 million to develop the vaccine.
What Next?
This current version of Mosquirix is not expected to be the last. Preliminary results for a new modified vaccine, called R21, are encouraging.
Other malaria vaccines in development include whole parasite vaccines. These use the whole malaria parasite that has been killed or altered so it cannot cause a malaria infection but can still stimulate an immune response.
Passive vaccines are also being investigated. These involve injecting long-lasting antibodies to prevent malaria infection.
A Whole New Set Of Challenges
In the meantime, WHO’s recommendation presents a new set of challenges.
Malaria-affected countries must decide whether to include Mosquirix as part of their national malaria control strategy.
Critical funding decisions from the global public health community will be needed to enable a broad rollout of the vaccine to the children who will most benefit from it.
Manufacturing capacity for tens of millions of doses each year, global vaccine supply chains and distribution infrastructure in malaria-affected countries will also be needed.
Finally, each country will need to maximise vaccine uptake and ensure completion of the four-dose immunisation schedule to obtain the vaccine’s full benefit.![]()
Danielle Stanisic, Associate Research Leader, Institute for Glycomics, Griffith University and Michael Good, Professor and NHMRC Investigator Fellow, Institute for Glycomics, Griffith University
This article is republished from The Conversation under a Creative Commons license. Read the original article.